Resources for verifying the legitimacy of customers and collaborators
Challenge: rigorous yet inclusive managed access to risky materials
Many providers already conduct KYC screening to decide whether to sell or share materials such as synthetic DNA encoding pathogens and toxins. However, it is not easy to design screening processes that are consistent and rigorous enough to capture potential illegitimate actors while enabling legitimate bioscience and biotechnology. This problem becomes especially apparent when trying to serve customers and collaborators outside of the Global North and established academic settings.
At present, KYC screening often relies on subjective judgments about “legitimacy.” For this reason, IBBIS has engaged in consultations with global stakeholders to develop structured templates and flowcharts for requesting information and verifying legitimacy.
Our Work: practical tools for verifying legitimacy for DNA synthesis
The decision about whether to fulfill an order for synthetic DNA hinges on a judgement about whether the customer should be trusted to receive those sequences. Orders for potentially risky sequences require a higher level of confidence in the customer, meaning that sequence screening and customer screening both feed into the decision.
As part of our Common Mechanism project, IBBIS provides a freely available tool to identify sequences of concern, and has worked to develop practical resources for customer screening to complement this.
Forms and Decision Support for DNA Providers
To support KYC practices for providers of synthetic DNA, IBBIS has developed customer screening forms and decision support resources. These documents are based on international discussions of best practices among these providers, guidance from U.S. and U.K. governments, and feedback from stakeholders.
Refinement of these documents is ongoing, and we would be very interested to hear how they could be improved to better accommodate a wider range of international providers and customers. If you have comments or suggestions, please send us an email at screening@ibbis.bio.
Onboarding New Customers
Customer screening can occur across many parts of the order process, but one key checkpoints for verification of legitimacy are during initial customer onboarding. We expect this form would be filled out just once, when a customer creates an account with a synthesis provider.
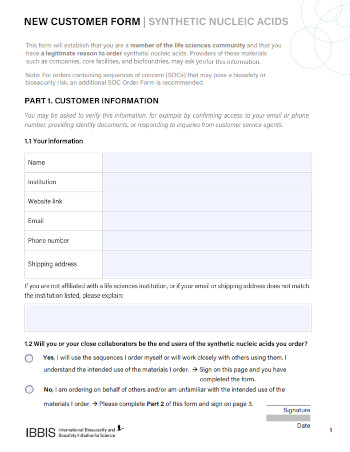
New Customer Form
The information on this form should be requested during customer onboarding. It will establish that customers are members of the life sciences community and that have a legitimate reason to order synthetic nucleic acids.
This form is available as a fillable PDF, word document, or plain text.
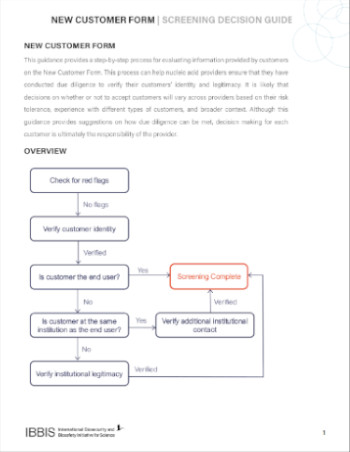
Decision Support for Providers
This decision guide outlines a step-by-step process for providers, such as companies, core facilities, and biofoundries, to decisions based on the information provided on the New Customer Form. This includes how to interpret information on the form, strategies to verify customer identity, and potential red flags.
Orders for Sequences of Concern
Additional customer screening is warranted for orders containing sequences that contribute to pathogenicity, virulence, or toxicity.
SOC Order Form
This form will help establish that customers have legitimate use and appropriate biorisk management when ordering sequences of concern (SOCs). The form asks for information about the order, its intended end use, and institutional approvals or other biorisk management practices.
This form is available as a fillable PDF, word document, or plain text.
Decision Support for Providers
This decision guide outlines a step-by-step process for providers to decide whether to provide an sequence of concern based on information provided on the SOC Order Form. This includes how to interpret information on the form, strategies to verify customer identity and legitimacy, and potential red flags.
Order Screening Game
IBBIS has developed a series of customer profiles based on real examples of legitimate scientists and attempted bioterror. They aim to illustrate some of the challenges inherent to verifying customer legitimacy for providers of custom synthetic nucleic acids.
The profiles have been used in interactive screening exercises by synthesis providers and during workshops hosted by the Global Biofoundries Alliance, iGEM Competition, Engineering Biology Research Consortium, SynBioBR, and others.
IBBIS is happy to run this exercise for interested providers, but we have also made the workshop materials available under CC BY-ND 4.0; we grant additional permission for others to translate the personas, provided the translated material maintains fidelity to the original content and is not otherwise altered.
- Workshop slides: ibbis.bio/customer-screening-workshop-slides
- Customer profiles: ibbis.bio/customer-profiles-for-screening

Publications
- August 2025. Implementing Emerging Customer Screening Standards for Nucleic Acid Synthesis. Sarah R. Carter, Lucas Boldrini, Tessa Alexanian. IBBIS White Paper. Available online.
- February 2024. Verifying Legitimacy: Findings from the Customer Screening Working Group, 2020-2023. Tessa Alexanian, Sarah R. Carter. IBBIS White Paper. Available online.
- January 2020. Biosecurity Innovation and Risk Reduction: A Global Framework for Accessible, Safe and Secure DNA Synthesis. Elizabeth Cameron, Sarah R. Carter, Jacob Jordan, and Ryan Morhard. Report of the NTI-WEF Working Group on Working Group on Preventing Illicit Gene Synthesis. Available online.